PHOTOBIOMODULATION
WHAT IS A PHOTOBIOMODULATION
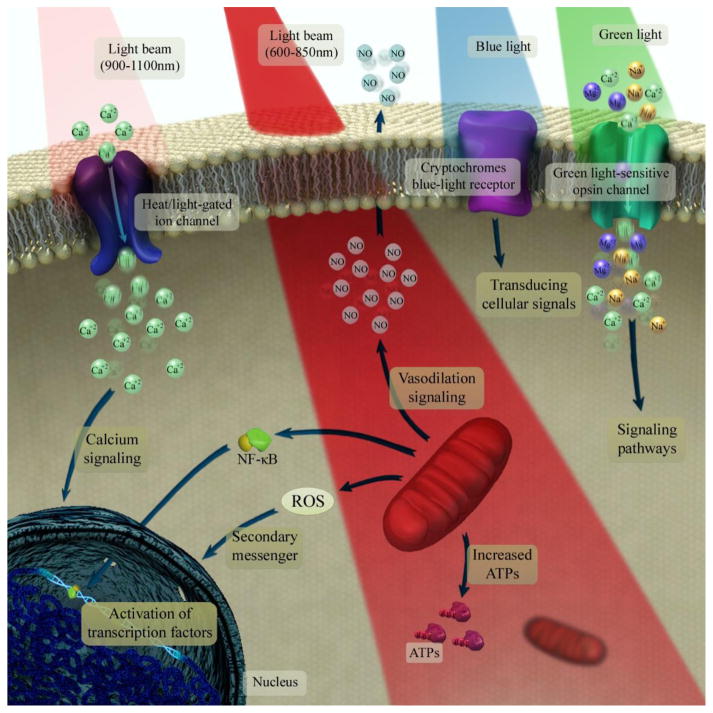
Brain photobiomodulation (PBM) therapy using red to near-infrared (NIR) light is an innovative treatment for a wide range of neurological and psychological conditions. Red/NIR light is able to stimulate the mitochondrial respiration (cytochrome c oxidase) in a wide range of cells and tissues in the human body1,2,3,4. .
Essentially the Mitochondria absorb the light which is then converted into ATP (energy source) for use within the cell. light absorption by ion channels also results in release of Ca2+ and leads to activation of transcription factors and gene expression and increased oxygen absorption. Transcranial photobiomodulation (tPBM) is a type of PBM that delivers NIR light/laser to the human brain, that enhances the metabolic capacity of neurons and stimulates anti-inflammatory, anti-apoptotic (cell death), and antioxidant responses, as well as neurogenesis and synaptogenesis (cellular generation and repair) within the brain itself. This emerging therapy has shown promising outcomes in treating psychiatric and neurological disorders5, such as depression and anxiety6, and traumatic brain injuries7,8.
Recent studies have reported that tPBM with a 1064-nm laser can enhance human cognitive performance on a variety of cognitive tasks using sham-controlled experiments9,10,11,12,13. More recently it has been demonstrated that 1064-nm tPBM enabled significant upregulation in concentrations of hemoglobin oxygenation ([HbO]) and oxidized cytochrome-c-oxidase ([CCO]) during and after tPBM on the human right forehead with high reproducibility and robustness14,15,16. These findings supported and validated the hypothesized mechanism of action that tPBM can photo-oxidize CCO, the key mitochondrial enzyme for cellular oxygen metabolism, to boost the metabolic activities of cells17, especially neurons18
Eeg and photobiomodulation
Recently, results revealed that tPBM is effective in enhancing EEG alpha and beta rhythms in the human brain during eyes-opened resting state, measured by 64-channel scalp EEG from healthy humans20,21. Similar observations on EEG responses to tPBM were reported by other groups while using different experimental protocols22,23,24. All these studies consistently indicated that tPBM can also modulate neuronal or electrophysiological synchronization and connectivity in the human brain.
Neural networks in the brain give rise to electrical signals that are known as brainwaves. These brainwaves drive the various networks in the brain that control attention, memory, filtering irrelevant information, and thoughts to name a few and ultimately, our behaviour and emotions.
Brain stimulation via photobiomodulation as described above results in improved efficiency in neural signalling and communication and can alter the Eeg.Vielight, pioneers in the field, have conducted research on their product and demonstrate the ability to modulate and alter brainwaves using NIR electromagnetic (light) energy, measured through EEG.
• Alpha is the resting state for the brain, an idling state and is present during calm and some medicative states. Initial EEG testing with the Vielight that pulses the light at Alpha’s 10 Hz demonstrated that it can elevate neural Alpha waves.
• Gamma is the active state of the brain and mostly prominent when the brain is attentive and/or learning and in complex cognitive processes. Initial EEG testing pulsing at a 40 Hz pulse can elevate neural Gamma waves.
The study by Wang et al20 demonstrated that tPBM with a 1064-nm laser given on the right forehead of healthy human subjects neuromodulated delta, alpha, and beta oscillations in eyes-closed resting state. The observed significant enhancement on alpha and beta powers by tPBM in the anterior–posterior regions may be the underlying electrophysiological mechanism of action to explain why tPBM enables the improvement of human cognition.
Qeeg guided tpbm
Neuroperformance uses Qeeg to inform and support most of its treatments. Photobiomodulation is delivered using the new, cutting edge iSyncWave Qeeg helmet which also delivers the photobiomodulation. This device is the most flexible on the market meaning that following a Qeeg, the frequency at which and the locations to which the light therapy is provided can be tailored to each individual. All treatments with tPBM are preceded and informed by a Qeeg and will include repeat ‘brain scans’ to monitor treatment response.
At Neuroperformance, we also believe that any treatment should be delivered within the whole systems NEURO program approach and will also include a focus on nutraceuticals, lifestyle and diet changes that may support the brains responsiveness to the treatment.
Who may benefit using tpbm?
Neuromodulation treatments like tPBM are still in their infancy research wise. However, clinically and with the limited research already todate, it appears that those who have conditions that are associated with neuroinflammation are likely to gain some benefit. The safety profile is also well established.
A number of clinical trials are using tPBM for Alzheimers and traumatic brain injury as well as to improve cognitive performance. tPBM may well benefit those clients who are seeking to address these issues. A Qeeg and thorough assessment will determine if this is the right treatment for you. tPBM is also commonly at neurofeperformance combined with neurofeedback to enhance the neural plasticity of the brain thereby increasing the chances of the neurofeedback being more effective.
1. Chung, H. et al. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 40, 516–533. https://doi.org/10.1007/s10439-011-0454-7 (2012).
2. Wong-Riley, M. T. T. et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 280, 4761–4771 (2005).
3. Gonzalez-Lima, F. & Barrett, D. W. Augmentation of cognitive brain functions with transcranial lasers. Front. Syst. Neurosci. 8, 36. https://doi.org/10.3389/fnsys.2014.00036 (2014).
CAS Article PubMed PubMed Central Google Scholar
4. Hamblin, M. R. In Photobiomodulation in the Brain (eds Hamblin, M. R. & Huang, Y. Y.) (Academic Press, 2019).
5. Rojas, J. C. & Gonzalez-Lima, F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem. Pharmacol. 86, 447–457. https://doi.org/10.1016/j.bcp.2013.06.012 (2013).
CAS Article PubMed Google Scholar
6. Cassano, P., Petrie, S. R., Hamblin, M. R., Henderson, T. A. & Iosifescu, D. V. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 3, 031404. https://doi.org/10.1117/1.NPh.3.3.031404 (2016).
7. Naeser, M. A., Saltmarche, A., Krengel, M. H., Hamblin, M. R. & Knight, J. A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed. Laser Surg. 29, 351–358. https://doi.org/10.1089/pho.2010.2814 (2011).
Article PubMed PubMed Central Google Scholar
8. Naeser, M. A. et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J. Neurotrauma 31, 1008–1017. https://doi.org/10.1089/neu.2013.3244 (2014).
Article PubMed PubMed Central Google Scholar
9. Barrett, D. W. & Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230, 13–23. https://doi.org/10.1016/j.neuroscience.2012.11.016 (2013).
CAS Article PubMed Google Scholar
10. Blanco, N. J., Saucedo, C. L. & Gonzalez-Lima, F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol. Learn. Mem. 139, 69–75. https://doi.org/10.1016/j.nlm.2016.12.016 (2017).
11. Blanco, N. J., Maddox, W. T. & Gonzalez-Lima, F. Improving executive function using transcranial infrared laser stimulation. J. Neuropsychol. 11, 14–25. https://doi.org/10.1111/jnp.12074 (2017).
12. Vargas, E. et al. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 32, 1153–1162. https://doi.org/10.1007/s10103-017-2221-y (2017).
Article PubMed PubMed Central Google Scholar
13. O’Donnell, C. M., Barrett, D. W., Fink, L. H., Garcia-Pittman, E. C. & Gonzalez-Lima, F. Transcranial infrared laser stimulation improves cognition in older bipolar patients: Proof of concept study. J. Geriatr. Psychiatry Neurol. https://doi.org/10.1177/0891988720988906 (2021).
14. Wang, X. et al. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 37, 3789–3802. https://doi.org/10.1177/0271678X17691783 (2017).
CAS Article PubMed PubMed Central Google Scholar
15. Wu, Q., Wang, X., Liu, H. & Zeng, L. Learning hemodynamic effect of transcranial infrared laser stimulation using longitudinal data analysis. IEEE J. Biomed. Health Inform. https://doi.org/10.1109/JBHI.2019.2951772 (2019).
Article PubMed PubMed Central Google Scholar
16. Pruitt, T. et al. Transcranial photobiomodulation (tPBM) with 1,064-nm laser to improve cerebral metabolism of the human brain in vivo. Lasers Surg. Med. https://doi.org/10.1002/lsm.23232 (2020).
Article PubMed PubMed Central Google Scholar
17. Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 49, 1–17. https://doi.org/10.1016/S1011-1344(98)00219-X (1999).
CAS Article PubMed Google Scholar
18. Rojas, J. C. & Gonzalez-Lima, F. Low-level light therapy of the eye and brain. Eye Brain 3, 49–67. https://doi.org/10.2147/EB.S21391 (2011).
Article PubMed PubMed Central Google Scholar
20. Wang, X. et al. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. NPh 6, 1. https://doi.org/10.1117/1.NPh.6.2.025013 (2019).
21. Wang, X., Dmochowski, J., Husain, M., Gonzalez-Lima, F. & Liu, H. Proceedings #18. Transcranial infrared brain stimulation modulates EEG alpha power. Brain Stimul. 10, e67–e69. https://doi.org/10.1016/j.brs.2017.04.111 (2017).
22. Spera, V. et al. Transcranial near-infrared light: Dose-dependent effects on EEG oscillations but not cerebral blood flow. bioRxiv https://doi.org/10.1101/837591 (2019).
23. Berman, M. H., Hamblin, M. R. & Chazot, P. In Rhythmic Stimulation Procedures in Neuromodulation (eds Evans, J. R. & Turner, R. P.) 97–129 (Academic Press, 2017).
24. Zomorrodi, R., Loheswaran, G., Pushparaj, A. & Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Sci. Rep. 9, 6309. https://doi.org/10.1038/s41598-019-42693-x (2019).



